MORE ENERGY OF PHOTOSYNTHESIS
 |
||
|
By Dr. Lalit Chudiwala, Independent Researcher |
FIELD OF INVENTION
Photosensitized Decomposition of methane under Specific Heat, Light and Vacuum Conditions. This is a Free Radical Chain Reaction of Mercury and Methane, in Which Mercury is used as a Catalyst and Provides Alternate Pathway of Lower Activation Energy. As a Result of Which High Amount of Energy is released. This Reaction Comprises of Four Steps: 1) Dissociation 2) Excitation 3) Ionization 4) Association
BACK GROUND OF INVENTION
This is a Free Radical Chain Reaction of Mercury and Methane in Which Input Energy is Low and High Amount of Energy is released. We Extract the Output Energy by Heat Exchangers and used it in Thermal Power Stations to Boiled Water for Generating Electricity. By This Reaction, By-Products We Get Are Ethane, Methane And Hydrogen Which Are Eco-Friendly. So there is No Wastage of Fuel and is Economical As Well.
SUMMARY
Mercury Photosensitization is one of the cornerstones of Photochemistry, yet many of the details of mechanistic pathway involved remain obscure. A number of points concerning mercury photosensitized reactions are widely accepted.
In the first step of process,( Ref 1 ) mercury atom in the reactor absorbs 265.6 nm photon from mercury lamp. the ground state of mercury is excited to 3P1 state , commonly referred as Hg*which has a life time of 1.1 × 10-7 S-1. Hg* forms exciplexes with a variety of molecules which may either luminescence and dissociate or more commonly exciplexes undergoes chemical reaction.
Hg* Exciplexes is formed with reaction chamber binding energy of 2.6 K Cal /mol which is slightly less than corresponding molecular hydrogen complex binding energy of 3K Cal/mol . The value of methane compares favourably with the experimental value of 2.0 K Cal/mol which best supports the assumption that spin-orbit effects are not quenched for these weakly bound exciplexes.
(Ref 2) the methane group, on the other hand has a directed bond which means that methyl-hydrogen bond in methane must be broken to some extent and the methyl group tilted before methyl radical orbital can start to overlap efficiently with metal orbitals.
(Ref 3) mercury photosensitized decomposition of methane is a well-supported method for generation of atomic hydrogen. The efficiency of this reaction close to unity and proceeds through simple mechanism as show in the equation.
Hg* + R-H ------- Hg + R + H (photosensitized mercury )
When methane gas is subjected to decompose in presence of Hg in excited state. The energy is transferred to CH4 for Free radical formation. The possible mechanism of the reaction
CH4 + Hg*-------- CH3*+ H*+ Hg*
In this reaction, Hg* excited state is sufficient to break C-H bond. In this reaction , light intensity must be low enough so that loss of hydrogen atoms and radicals recombination reaction which complete with reactions i.e.:
CH3* + H2 ------- CH4 + H*
H* + CH4 ------- H2 + CH3*
These reactions are favoured by high intensity and high radical concentration may be neglected. Concentration of products and impurities must be kept low enough so that only methane quenches mercury atoms and some secondary reactions occurs in such a way.
H2 + CH3* ------ CH4 + H*
Last step is termination step which involves the formation of ethane and hydrogen as a by-products which are eco-friendly and can be reused again in mercury photosensitizes reaction. The possible reactions are as follows:
CH3* + CH3* ------ C2H6
H* + H* ------ H2
CH3* + H* ----- CH4
Hydrogen is the only by-product measured is most of the experiments. In few instances, formation of ethane and methane are observed. In all the experiments, concentration of mercury to hydrogen was negligible. The yield of hydrogen observed to be independent of conversion both in single experiment and in sequence of experiments starting with pure methane and allowing ethane to accumulate. In these reactions, neither hydrogen nor ethane was quenching mercury atoms as was affecting hydrogen yield in any other way.
In the above reactions, temperature may be increased due to recombination of H-atom radical. Also in the above reactions, the effect of added hydrogen is explained by increase in rate of recombination of hydrogen atoms (Ref 4) the interaction of methane with deuterium has also been observed under the influence of photosensitized mercury. The influence of temperature, light intensity and mercury concentration on yield of deutromethanes has been measured. Below 200*C, the result indicates the production of deutromethanes is governed by photosensitized formation of free methyl radicals. At high temperature, these chain reactions become important and high quantum efficiencies are observed.
Conditions for the reactions to occur efficiently
- Temperature should be greater than 120*C
- Pressure should be above 590mm of Hg
- Frequency of light should be preferably 2656A* for the excitation of Hg molecule.
- Preferably Hg source is used. Even flash lamp can work but it should be operated on 60 cycles/sec
Energy released is in the form of radical Recombination and this large amount of energy generated due to chain reactions. Chain reactions and H molecule or cation of H generation is indicated and that released energy is used to boil water for generating electricity.
FREE RADICAL CHAIN REACTION OF METHANE
TABLE 1
|
REACTION |
|
ENERGY |
|
CH4 + Heat = CH3* + H* |
R-1 |
R-1 = 104.74 + K/Cal |
|
CH3* + H2 = CH4 + H* H* + CH4 = H2 + CH3* H2 + CH3 = CH4 + H* |
R-2 |
R-2 = 16.26 + K/Cal |
|
CH3* + CH3* = C2H6 |
R-3 |
R-3 = 86.5 - K/Cal |
|
H* + H* = H2 |
R-4 |
R-4 = 103.75 - K/Cal |
|
|
|
Net Profit = 69.25 K/Cal/per molecule |
(+) Absorbed
(-) Released
TABLE 2
|
|
Activation Energy |
Arrhenius Coefficient |
Rate constant Cm3/Molecule/Sec |
Temp. K* |
|
R-1 |
104.89 K/Cal |
1.56 E + 16 |
16.8791904 |
1500 K* |
|
R-2 |
3.557 K/Cal |
1.56 E - 12 |
3.07434 E-12 |
1500 K* |
|
R-3 |
0.679388 K/Cal |
1.00 E - 14 |
1.26072 E-14 |
1500 K* |
|
|
|
|
|
|
|
|
|
|
|
|
|
R-1 |
104.89 K/Cal |
1.56 E + 16 |
0.0580615 |
1300 K* |
|
R-2 |
3.557 K/Cal |
1.56 E - 12 |
1.32022 E-12 |
1300 K* |
|
R-3 |
0.679388 K/Cal |
1.00 E - 14 |
1.03646 E-14 |
1300 K* |
|
|
|
|
|
|
|
|
|
|
|
|
|
R-1 |
104.89 K/Cal |
1.56 E + 16 |
2.6708 E-05 |
1100 K* |
|
R-2 |
3.557 K/Cal |
1.56 E - 12 |
4.74727 E-13 |
1100 K* |
|
R-3 |
0.679388 K/Cal |
1.00 E - 14 |
8.18956 E-15 |
1100 K* |
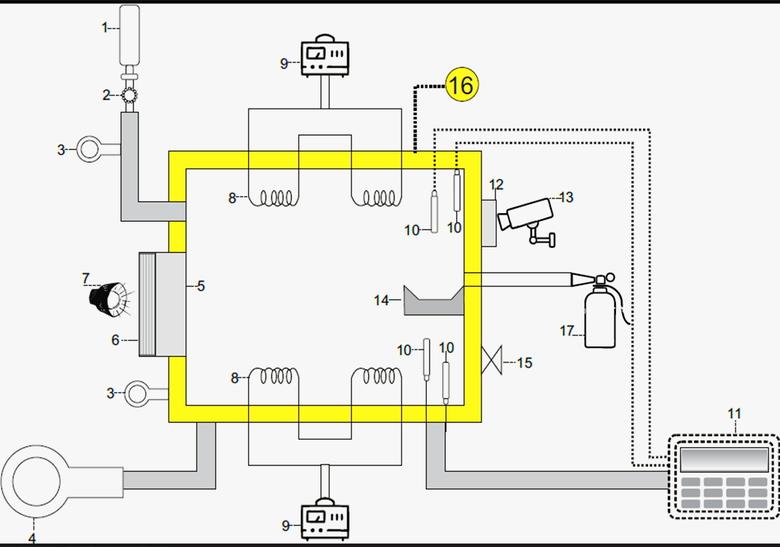
1. Methane cylinder (STP = Standard Temp./Pressure) or NTP. 1 Mole Methane 16g 22.4 ltr.
2. Gas Inlet Valve (Manual or auto)
3. Pressure Monitor/Gauge (Digital / Analogue)
4. Roughing Pump to achieve vacuum 10-3 bar
5. Transparent Window (Quartz / perpex)
6. Shutter for Mercury Resonance Lamp
7. Mercury Resonance Lamp 2656 Ao
8. 2 Pair Tungsten/ Iridium Filament to heat up the reaction chamber and gas upto a desired temp. of 200o to 2000oc.
9. DC supply & circuit for the heater filaments (Variable High current supply).
10. Thermo-couples to measure temp. of chamber & gas.
11. Data Logger / display for Thermo Couples.
12. Observation Window /diagnostic window (Quartz / perpex)
13. Observation Camera / IR Camera (Thermal Measurement)
14. Mercury Stand
15. Chamber safety valve
16. SS Reaction Chamber
17. Co2 Quench Cylinder for safety or emergency.
Initial Reaction Concept Test Chamber ( Rectangular Chamber Cross Section )
1 Mole of Methane ( CH4 ) which weight 16g or 22.4L. The gas inlet Valve could be manual or automatic .Firstly , valve is open by using roughing pump to achieve vacuum (~ 10-3 bar ). The transparent window are made of quartz. A shutter is placed for mercury lamp of 2656 A*.Two Pair of Tungsten/Iridium Filament is used to heat up the vessel up to desired temperature of 200* C – 2000* C . For Variable high current supply, DC circuit is attached to heating Filaments . Data logger is used to display Thermocouple. Observation camera is used for thermal measurements. Mercury (Hg*) sample is placed on stand and should be set at such a valve to avoid breaking of chamber. A chamber safety valve is also placed with SS chamber. For emergency situations Co2 Cylinder is placed which quenches the reaction.
OPERATION OR WORKING
Close all the valves of the system. Open the valve attached with the roughing pump and start the pump. Slowly achieve rough vacuum level. Now open the gas inlet valve to fill required amount of fuel, note the chamber pressure and close the valve. Switch on the heater and observe the temperature on the data logger. The current in the heater can be decided on the basis of the saturation temperature. The temperature inside the chamber can be verified using the IR camera by anodized black inner wall of the chamber.
Note the pressure inside the chamber after reaching the required temperature. Before filling the chamber with fuel, it must go through this kind of boiling cycle 3 times to finally start the process. Now switch on the Mercury lamp with moderate intensity and open the shutter in front of the lamp. At this point, the reaction must start, and the mercury sample must be in the line of sight of the lamp light. Keep monitoring the pressure and the temperature inside the chamber simultaneously. If these two go out of control, immediately close the shutter as it might be dangerous. If ionization initiates properly, bright light will be observed inside the chamber and the experiment will be successfully completed.
Demonstration Prototype
On the most basic level, the machine consists of an SS Chamber which is also used for “Reaction concept test” with all the auxiliaries as they were in it. Moreover, two new connections are added for the “gas flow circuit”. A Blower is connected with the reaction chamber in a closed loop circuit which is VFD controlled. A heat exchanger plate or a cold plate is attached to the system. A valve is placed in the closed loop and a water tank is put. A motor is also used which is VFD controlled. For the detailed measurements, an Energy meter (∆E = mg∆T) is used. A thermocouple and a flow monitor is attached to the system as the input energy meter. As a safety thermostat during any emergency, a temperature monitor is placed. For the residual fuel, the by-product confinement chamber is created. The machine also consists of two control valves and a roughing pump.
RESULTS AND DISCUSSIONS
The possible reaction paths to the release of H-atom as “radical” in chain reactions has been figured in Table 1. The corresponding reactions rates and the activation energies at various temperatures are reported in Table 2. It can be seen from Table 2 that the reaction R-3 (see Table 1) has the least activation energy and R-1 has the maximum.
In chemical kinetic study the fundamental rule is that higher is the activation energy, the feasibility of that reaction is difficult means the reactant converted into product is least. Whereas, the least activation energy makes the reaction much faster to give more products.
Hence, it can be concluded from the above results, R-3 and R-4 reactions are feasible and can produce the required product on H2 gas.
REFERENCES
1) Yale Chemistry Dept., New Haven CT, 06511, USA
Pure &Appl. Chern., Vol. 67, No. 1, pp. 39-45, 1995.
2) Por E.M.siegbahn,mats svensson, and Robert H. Crabtree
J. Am. Chem. SOC. 1995,117, 6758-6765
3) R.A.Back and D. Vander Auwera (1962)
Can. J. Chem. 1962.40:2339-2347.
4) R.Berisford and D.J.Le Roy
Can. J. Chem. 1958.36:983-989.
-----
Earlier: