RADIATED ENERGY FOR CLIMATE
Radiated Energy and the Second Law of Thermodynamics
Douglas Cotton, B.Sc., B.A., Dip. Bus. Admin
March 12, 2012
ABSTRACT
The transfer of thermal energy by radiation is discussed in the context of the Earth's surface and its atmosphere. When considering what happens as the Sun is warming the surface each morning, it is noted that its radiation is being directed onto the land surfaces and some distance below the surface of the oceans. So, additional radiation supposedly transferring further thermal energy from the cooler atmosphere to the warmer surface would violate the Second Law of Thermodynamics. This law must apply (on a macro scale) between any two points at any particular time. An apparent
violation cannot be excused on the basis of "net" radiation, because "net" radiation has no corresponding physical entity and is meaningless and useless for determining heat flow in situations when other processes are also involved.
It may be deduced that none of the radiation from a cooler body (and only a portion of the radiation from a warmer body) has any thermodynamic effect on the other body. All such radiation from a cooler source is rejected in some way, and it can be deduced that resonance and scattering occurs without any conversion to thermal energy. The radiation continues in another direction until it strikes a cooler target, which could be in space.
Furthermore, the stability of sub-surface temperatures will tend to maintain the observed close thermal equilibrium at the interface between the surface and the atmosphere. Hence other heat loss mechanisms are likely to adjust, in order to
compensate for any reduced radiation.
Some commonly raised questions are answered in the Appendix, where there is also discussion of temperature trends and climate cycles, as well as counter arguments for several possible objections to matters raised herein.
1. Introduction and terminology
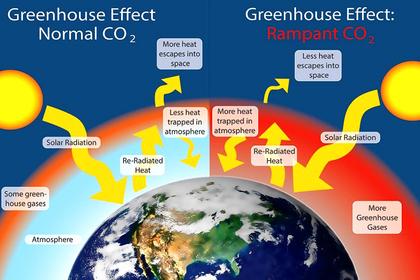
Originally it was thought that the Earth's atmosphere acted like a “blanket” and that trace molecules like carbon dioxide helped to absorb radiation and trap “heat” which would then somehow warm the surface. Carbon dioxide represents about one molecule in over 2,500 other molecules and it (together with about 20 to 50 times as many water vapour molecules and some other trace gases) is, in fact, able to capture “photons” and radiate energy away to space. These gases can even absorb some of the incoming infra-red solar radiation. By reflecting and absorbing some incident solar radiation, the atmosphere does indeed keep the Earth's surface cooler in daylight hours.
Furthermore, there is a long-term close thermal equilibrium between it and the surface, which has been established over some four billion years. Fortunately the crust and mantle beneath it act as very good insulators, retaining thermal energy in the core and only allowing a trickle to leak out. This ensures long-term stability of temperatures, even just a few metres below the surface, and that in turn helps to maintain stability in surface and lower atmosphere temperatures. As a result, the mean of such temperatures (when calculated over 60 years) tends to vary little more than about 2oC above or below the thousand year mean.
But, just as a vacuum flask does not further warm the coffee, neither does any additional temporary thermal energy trapped by the atmosphere warm the surface. Such energy may perhaps “warm up” the atmosphere a little to, say, -35oC or some such temperature well below freezing, but the real insulation property of the atmosphere has more to do with the rate at which warm air rises and creates an inevitable temperature gradient.
So when these original “greenhouse” conjectures (devised by climate scientists) came under the scrutiny of physicists, it became apparent that warm air rises rather than falls, and that any excess trapped “heat” (as they mistakenly called it) would simply be radiated away pretty quickly. So then, in the early 1980's, they had to turn to “Radiative Transfer Theory” and ensure that radiated energy could be seen to dominate the whole process. So they suggested that radiation from the cooler atmosphere would further warm the surface as it made its way up and down, numerous times it seems, dropping off a bit of “heat” on every visit.
But climate scientists have erred in thinking that any “thermal” radiation can add thermal energy to the surface, regardless of the temperature of the surface. This mistaken belief originates from visualising radiation as a flow of mass-less “photons” colliding with molecules in the surface and automatically warming them, if the photons were not reflected beforehand.
There is a need to clarify the fact that “heat” is not automatically transferred wherever “thermal radiation” flows. The very term “thermal radiation” is misleading because it may be interpreted as meaning radiation only in the infra-red spectrum. But these are not the only wavelengths which can bring about a transfer of thermal energy, which may be thought of as a heating process. Solar radiation is nearly half made up of radiation in the infra-red, but the rest in the visible light and ultra-violet spectra can and does transfer even more thermal energy, which warms the surface of the Earth.
For example, when light strikes a yellow target the radiation for red, violet etc will not be reflected and will usually be converted to thermal energy. Ultra-violet light has a strong warming influence and our skin will react, especially when the UV index is high in summer.
So we should not speak of rays of “light” or “thermal radiation” but rather just “radiated energy” for such is neither light nor heat. This is because we do not know exactly what will happen to the radiated energy contained in any particular ray until it strikes a target. There it may be reflected either as spectral (mirror-like) reflection or as diffuse (scattered) reflection.
If it is not reflected it may be transmitted through glass, for example, or absorbed. But the following discussion will point to the need for another different process that must exist in order to explain observations, and to provide a mechanism whereby nature ensures that the Second Law of Thermodynamics is not violated.
2. Does radiation transfer heat in both directions simultaneously?
It is well known that, when two parallel plates at different temperatures radiate towards each other, the warmer one cools and the cooler one warms until the temperatures are equal. So there appears to be some feedback mechanism, but is it really a two-way heat transfer?
If thermal energy could transfer from cold to hot, then what happens when radiation from the atmosphere penetrates some small distance into the ocean waters? Does it warm the water which then rises to the surface by convection and causes more evaporation? Such a scenario can not be right and the only feasible explanation is that, even though there may be two-way radiated energy transfer, the radiation from the cooler body to the warmer one cannot be absorbed and converted to thermal energy when it reaches the warmer body. This is the conclusion drawn by Professor Claes Johnson in his Computational Blackbody Radiation where he suggests that such radiation merely resonates with the warmer body.
When calculations are done to estimate the amount of radiation (radiative flux) between two plates, it is normal to use the Stefan-Boltzmann Law to calculate the flux in each direction and assume that the difference is “net” radiation, from which is derived “net heat flow.”
It is further assumed that, because this net radiation is in the same direction as the observed heat flow (from the warmer plate to the cooler one) then there is no violation of the Second Law of Thermodynamics, which says this is how it should be.
But does the radiation in each direction somehow cancel out to give a “net” radiative flux in the direction of the heat transfer, or are there still two distinct radiation beams? Or could there be a separate transfer of thermal energy in each direction leading to a net effect?
If you have a beam of sunlight coming into your room you can still shine torch light right through the sunlight and see the effect of the torch light on the wall. It is not affected in any way if the Sun's rays are then blocked. In the atmosphere are numerous radiation beams for radio broadcasts and television transmissions, but they do not affect each other. So radiation with different directions and different wavelengths does not appear to combine into any “net” radiation with a net radiative flux, transferring a net amount of thermal energy.
When the Sun is warming the surface on a clear morning at, say, 11.00am we know that there is a net energy inflow into the surface, simply because it is getting hotter. This heat transfer is calculated even after deducting the outflow, which may be a mixture of radiation, evaporative cooling, chemical processes, conduction or diffusion followed by convection.
What happens if we then “add” radiation from the cooler atmosphere into the surface? This process is clearly independent of the solar radiation and other transfers of thermal energy out of the surface.
The models used by the UN Intergovernmental Panel on Climate Change (IPCC) assume this happens 24 hours a day, and that it either increases the warming rate in the morning, for example, or decreases the cooling rate later in the 24 hour cycle. But, when the surface is already warming and there is a net inward flow of energy, then clearly such radiation from the cooler atmosphere cannot transfer thermal energy to the warmer surface, for to do so would amount to heat flow from cool to warm, which violates the Second Law of Thermodynamics.
The Second Law must apply between any two points for each and every separate transfer of thermal energy at any given time. Clearly, if radiation from the atmosphere were to penetrate a small distance into an ocean and then be converted to thermal energy beneath the surface, there is no question of any simultaneous “balancing” flow of thermal energy out of the surface. Even if it took only a second for the warm water to rise and evaporate, this would be a separate process with no “memory” of the first one. You cannot say the Second Law is obeyed because of any subsequent transfer of thermal energy in the other direction. It would be like parking your car in a one hour spot for 80 minutes, and then in another spot for only 20 minutes, hoping you did not break the law because your average stay was 50 minutes.
So the assumptions of current physics that either radiation is compounded into “net” radiation, or that thermal energy is transferred each way must be incorrect. Neither happens.
3. What physical mechanism must determine if thermal energy is transferred?
We have discussed why radiation with different frequencies and directions cannot be compounded into “net” radiation. Indeed, energy which transfers into the oceans may well exit by evaporation rather than radiation. So the mechanism we seek cannot be one involving the compounding of radiation in order to produce a net transfer of thermal energy.
Only thermal energy can be compounded, not radiation. Adding thermal energy to the surface while it is cooling would indeed slow the rate of cooling, but any such “transaction” would be an independent process which would have to occur prior to that energy transferring back to the atmosphere by another process. Hence any such transfer of thermal energy from the atmosphere to the warmer surface must be in violation of the Second Law.
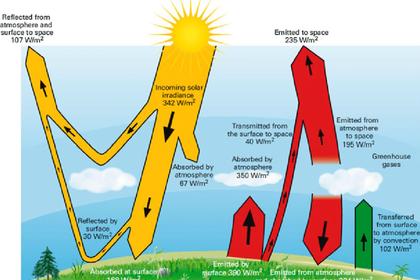
We need to consider the spontaneous radiation from a perfect blackbody, which has a frequency distribution with a shape such as that in each plot below. Here, for the horizontal axis, frequency has been chosen in order to demonstrate Wien's Displacement Law, which states that the peak frequency (the mode) is proportional to the absolute temperature. The shape of these curves thus looks different from those usually shown when discussing Planck's Law, because they are plotted against frequency rather than wavelength.
The total radiative flux is represented by the area under the curve for any particular temperature, and Stefan-Boltzmann Law tells us it is proportional to the fourth power of the absolute temperature.
But can we just take the difference between the radiative fluxes from two opposing bodies (such as parallel metal plates) at different temperatures and determine the quantity and direction of the heat flow, if any? This usually works, but when additional heat transfer processes (such as solar radiation, evaporation, diffusion etc) are involved in the real world, these processes must be considered separately and each must obey the Second Law of Thermodynamics in its own right. We have already seen that there cannot be a physical transfer of thermal energy from cold to hot bodies under any circumstances, and flow in the opposite direction does not excuse the violation of the Second Law. In any event, in the real world some of the energy going from hot to cold may be transferred by processes other than radiation.
The only logical conclusion to draw from this is that the radiated energy from a cooler body to a warmer one is not being converted to thermal energy in the warmer one, so that the effective absorptivity must be zero. Only a portion of the radiation from the warmer body to the cooler one has any effect, and does all the “heating” work. So we need to quantify what is happening using only the radiation from hot to cold.
4. Quantification of one-way radiation causing heat flow
In calculating heat flow it is found that, for the case of two parallel plates, we get a satisfactory result by determining the radiative flux from each, then taking the difference to get net flux and determining heat flow from that. Such calculations of net flux effectively subtract the area under the curve of the cooler target from the area under the curve for the warmer source. So, in straight forward cases, the calculations are actually working with the area between the two curves.
Can any physical significance be placed upon this area between the curves if we are going to use just that area and consider only the radiation from the warmer source, disregarding the radiation from the cooler target?
It is indeed necessary to place a physical significance on such an area between the curves, if and only if it represents all the radiation from the warmer body which is actually absorbed by the cooler one. It must do just that, because that is the area which approaches zero when the temperatures approach each other. This area has a corresponding actual heat transfer, whereas the total areas under each curve do not.
Such a hypothesis requires the assumption that the portion of radiation from the warmer body which is represented by the area under the curve for the cooler one is all “rejected” by some physical process, and is thus not converted to thermal energy. An equivalent radiative flux from the cooler body to the warmer one is also not converted to thermal energy, but it does limit the amount by which the temperature of the warmer one can fall until both are at the same temperature. Obviously, if the warmer body's temperature were to fall below the cooler one, then the heat transfer reverses direction, because the latter would then be warmer.
5. The concept of resonant scattering
As quantum mechanics tells us, the electrons in molecules of matter can have various discrete energy levels. When they “drop” from a higher level to a lower one they emit a burst of radiation, referred to as a photon, which will now have the energy which the electron shed.
But radiation has a wavelike nature with a frequency which increases with the energy of each photon. Large numbers of molecules acting like this in a blackbody will emit radiation with a distribution of frequencies as we saw in the plots in Section 3. The peak frequency indicates the temperature of the emitting body. Hence, although one photon is not enough to determine the temperature, the frequency distribution of all the radiation does do so.
Now, if the warmer body cooled down to the temperature of the cooler one it would have a matching frequency distribution. We can postulate that there will be natural maximum frequencies with which the electrons can vibrate between energy states. So radiation with a matching frequency can resonate with molecules in the target. But the radiated energy in each photon is proportional to the frequency of the associated radiation, and that energy will be just the right amount to excite an electron to a particular higher state, but not enough to go the extra distance required for any of the radiated energy to be converted to thermal energy.
As this resonating process is taking place, a photon in the incident radiation excites an electron to a higher state, let's say at the crest of the wave, and then immediately lets it relax back to its original state at the trough. As it relaxes it sends an equivalent photon off in a different direction, thus seeming to scatter the initial radiation.
However, if one body is warmer than the other, then only that portion of the radiation which corresponds to the area under the smaller curve will experience resonant scattering, whilst the surplus (corresponding to the area between the curves) will be converted to thermal energy in the cooler body, thus warming it. The radiation which is scattered may well strike another target which is cooler again, and so a similar split of its energy could occur. If instead it meets a warmer target, possibly the Earth's surface, it will just undergo resonant scattering without leaving any energy behind. Eventually it will get to space where it will travel on for who-knows-how-long until it strikes another target, which also may be warmer or cooler. The “temperature” of the radiation is really just the temperature of a blackbody for which the peak frequency would be the frequency of the radiation.
If we have two large plates close to each other in parallel planes, then, near the centres of the plates, there would be significant radiation from the cooler plate to the warmer one. So this “backradiation” prevents the warmer one cooling below the temperature of the cooler one.
However, any radiation which reaches the Earth's surface from the atmosphere will have come from much cooler molecules and, in the real world, experiments such as that by Prof Nasif Nahle have shown that the atmosphere is usually cooler than the surface (even close to the ground) and it cools faster at night.
Hence, while the surface remains warmer than the base of the atmosphere, any radiation from the cooler atmosphere will undergo resonant scattering and this process leaves no additional thermal energy in the surface. So, under normal weather conditions, no thermal energy can be transferred from the atmosphere by radiation or any other spontaneous process.
In fairness, there would be a slight slowing of the rate of cooling when the temperatures approach each other, because of the way in which the area between the Planck curves reduces.
But this only applies to radiation, so evaporation and diffusion could easily compensate and it does not mean energy is added to the surface or the atmosphere.
6. Warming or Cooling Effects?
So we have seen that radiation from the atmosphere will usually be scattered after resonating with molecules on the surface, the end result being very much like diffuse reflection, though not technically the same. Only in fairly rare weather events would there be the possibility of warmer air existing just above the surface, and such air could warm the surface.
The most likely warming could be when water vapour close to the surface reaches higher temperatures in times of high humidity when the adiabatic lapse rate is also reduced. But water vapour also plays the major role in cooling the atmosphere by radiating away to space all the thermal energy which it acquires by diffusion in molecular collisions.
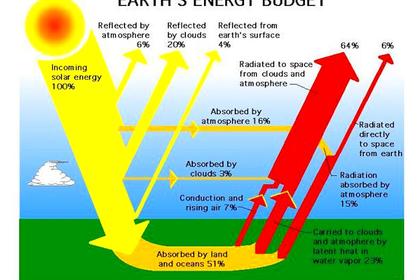
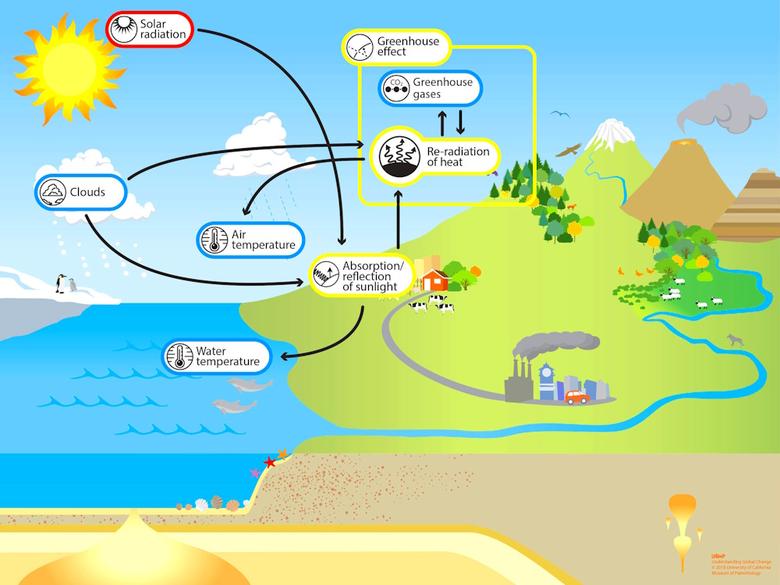
We need water for life but we also need it to moderate the climate. Water vapour cools the atmosphere by radiation and the ocean and earth surfaces by evaporation. It also reflects and absorbs some of the Sun's powerful incoming infra-red radiation, as may be seen below.
Carbon dioxide also absorbs incoming solar infra-red radiation and helps cool the atmosphere, radiating away to space not only the energy it captures from solar and surface radiation, but also that diffused from other air molecules. But, with its limited range of frequencies, it would not be very effective in slowing the rate of radiative cooling of the surface.
7. So where have the models gone wrong?
When scientists measure the “absorptivity” of a target material (which could be a gas, a liquid or a solid) they direct rays of visible light onto the target. They may well use photocells to measure the reflected and transmitted light, assuming the rest is the proportion absorbed and converted to thermal energy.
But how does this tie in with the Second Law of Thermodynamics? It cannot do so unless the absorptivity is a function of both the source and target temperatures. Furthermore, the absorptivity must reduce to zero whenever the temperature of the source no longer exceeds that of the target.
The models are based on calculating net radiation in and out of the surface. But we should not assume that we can perform vector-like compounding of rays of radiation, as we would for forces, and then deduce that heat flow is in the direction of this compound radiation. The Sun's radiation does not combine with the atmospheric radiation and, furthermore, there are other heat transfer processes involved.
Net radiation is meaningless for determining heat flow in some situations when other processes are also involved.
The models supporting the assumed greenhouse effect have all used incorrect values for absorptivity relating to the radiation from a cooler atmosphere to a warmer surface, because none of that radiation is absorbed and converted to thermal energy in the surface. The absorptivity for that radiation is zero.
8. Conclusion.
Consideration of the effect of the processes involved when the Sun is warming the Earth's surface in the morning leads to the logical conclusion that each such process must stand alone and not violate the Second Law of Thermodynamics. Thus radiation from a cooler atmosphere cannot transfer thermal energy to a warmer surface.
As a corollary, the absorptivity of spontaneous radiation from a cooler source to a warmer target must be zero.
As the assumption of a far greater absorptivity is inherent in the models and explanations of the so-called Atmospheric Greenhouse Effect (in which radiation from the atmosphere is assumed to warm the surface) such models and explanations do not reflect reality.
It is noted that radiation from the atmosphere can reduce the loss of thermal energy by the surface in rare situations related to weather conditions, usually in times of high relative humidity. But water vapour, as well as trace gases like carbon dioxide, can also have cooling effects absorbing some incoming solar infra-red radiation and radiating to space much of the thermal energy in the atmosphere.
-----
Earlier: